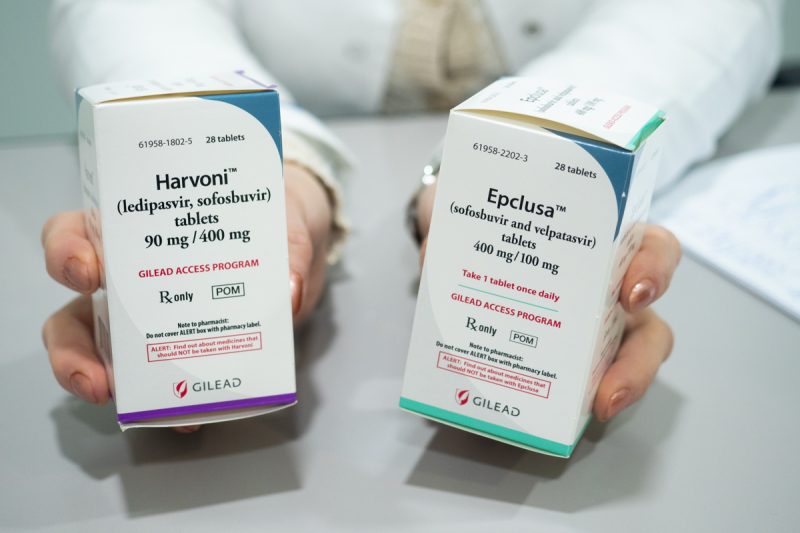
In a major win, the Department of Intellectual Property in Thailand satisfied the pre-grant opposition against patent application on sofosbuvir/ledipasvir (SOF/LED) in July 2024, which pertains to the drug sofosbuvir in combination with ledipasvir. This combination is recommended for treatment of genotypes 1, 4, 5 and 6 of chronic Hepatitis C.
This win comes after years of campaigning by the AIDS Access Foundation (AAF) and the Thai Network of People Living with HIV/AIDS (TNP+). Pharmaceutical giant Gilead initially filed sixteen patent applications in relation to SOF. Gilead decided to discontinue eight patent applications, and there were eight patent applications in the pipeline for patent examination. As a result, AAF and TNP+ filed six patent oppositions, two of which were third-party observations and four – as pre-grant oppositions. The opposition against Gilead’s patent application for the SOF/LED combination was initially filed in Thailand on April 3, 2018.
“This is one example of an evergreening patent because SOF/LED is a medicine that is available already. Everyone knows about the drugs SOF and LED — this combination just puts them both in one tablet. There is no inventive step,” said Chalermsak Kittitrakul, a researcher and social activist who is the Project Manager for TNP+.
Of the four pre-grant oppositions, three were rejected and one, which was regarding the SOF/LED combination, was accepted by the patent office. TNP+ filed appeals for the three rejected oppositions with their patent opposition team. Gilead is appealing the decision of Thai patent office about SOF/LED combination.
Patients with Hepatitis C in Thailand had been able to access the SOF/LED treatment at no charge since 2018. When a newer and more-effective treatment for chronic Hepatitis C was introduced and included in the National Essential Drug List, the SOF/LED regimen was then replaced by sofosbuvir/velpatasvir (SOF/VEL) in 2022.
Background on SOF in Thailand
In 2017, Thailand was included in Gilead’s voluntary license (VL) for sofosbuvir, allowing the import of generic versions, but this did not immediately resolve the issue of high prices. In 2018, SOF and the combination of SOF/LED was introduced in Thailand and included in the universal health coverage scheme’s benefit package. Thailand imported two generic versions from India, which led to a significant price reduction — from Gilead’s price of $1,100 USD per bottle for SOF to $110 USD.
In 2021, Thailand revised and simplified its standard of care for HCV to standard treatment of the pan-genotypic regimen of SOF and velpatasvir (VEL) supplied by generics manufacturers. In January 2021, three years after the WHO recommendation and years of campaign and advocacy work by TNP+ and AIDS Access Foundation led to sofosbuvir/velpatasvir (SOF/VEL) being added into the country’s National List of Essential Medicines. However, barriers remain, including restrictive eligibility criteria and the need for specialist care, which delay treatment and allow illnesses to worsen.
Although the VL allows Thailand to import generic versions of SOF/VEL from a licensee in India, it costs about USD 750 per treatment course. A local state-enterprise manufacturer could produce and supply generic SOF/VEL in 2022 at the price of USD 400 per treatment course.
The price for SOF/VEL remains unacceptably high because only one licensee filed for and obtained approval to market their product in Thailand and the local production requires the raw materials at high price from India. According to experts, generic versions of SOF/VEL could be profitably mass-produced for $86 USD per 12-week treatment course.
Efforts to Reform Thai Patent System
When it comes to protecting intellectual property, “There is a misuse and abuse of the [patent] system — instead of protecting and promoting innovation and access to lifesaving medicines, the system is protecting the multinational drug companies’ benefits,” Kittitrakul stated.
While the recent success of the SOF/LED opposition has marked a major milestone in the fight to keep medicines affordable and accessible, Kittitrakul stated there is much work to be done, including efforts to make the patent system in Thailand in balance of safeguarding public interest versus protecting the rights of patent holders through the Department of Intellectual Property (DIP), which manages intellectual property rights in the country.
“We want to make our patent system better and prevent it from granting this type of [evergreening] patent applications in their system. The DIP should consider that these types of patent applications should be rejected immediately in the examination process,” Kittitrakul stated, explaining that TNP+ is committed to ensuring pharmaceutical companies do not use evergreening as a tactic to monopolize the drug market and prevent access to life-saving and essential treatments for those who need them the most.
“To win this game, we have to make sure we have proper laws that see health before trade benefits,” said Kittitrakul, who added that Thailand is negotiating for free trade agreements (FTAs) with developed countries that demand Thailand to amend their intellectual property laws, including patent law, allowing to have longer and broader patent protection, accept ever-greening patents, and delay the competition of generic drug industry, and the multinational pharmaceutical corporations have been pushing this agenda through the free trade agreements.
In addition to these advocacy efforts, TNP+ is also focused on raising awareness on Hepatitis C within Thailand, particularly on informing individuals that it is a treatable and curable disease and the Thai patients can now get lab tests and treatment at no cost under the national health security schemes.