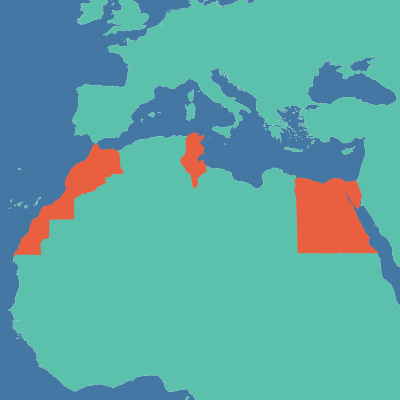
 On 1 December 2015 in Harare , at the International Conference on AIDS and STIs in Africa (ICASA), treatment advocates and activists from across Africa united to urge ViiV Healthcare to allow affordable access to an essential drug in four North African countries. ViiV’s voluntary license will enable access to generic versions of the antiretroviral drug, dolutegravir, for most countries in Africa, with the exception of Algeria, Libya, Morocco and Tunisia.
On 1 December 2015 in Harare , at the International Conference on AIDS and STIs in Africa (ICASA), treatment advocates and activists from across Africa united to urge ViiV Healthcare to allow affordable access to an essential drug in four North African countries. ViiV’s voluntary license will enable access to generic versions of the antiretroviral drug, dolutegravir, for most countries in Africa, with the exception of Algeria, Libya, Morocco and Tunisia.
On 30 November 2015, the World Health Organization (WHO) released its new treatment guidelines that recommend earlier treatment initiation for people living with HIV, and the use of dolutegravir, a more potent antiretroviral, with fewer side effects and a high genetic barrier to drug resistance.
“We welcome the new WHO guidelines that embrace current science and recommend early treatment initiation, as well as the use of a new generation of antiretrovirals,” said Mohammed Msefer, Regional Director of ITPC in the MENA region. “However, restrictions imposed by pharmaceutical companies like ViiV through patents and licensing strategies are a major obstacle to implementing the new guidelines in North African countries.”
ViiV and the Medicines Patent Pool issued a voluntary license on dolutegravir in 2014, allowing Indian manufacturers to supply 121 low-income countries with generic versions of the drug. All African countries are included in the license except these four North African countries.
Such exclusions increase the treatment gap… [and] condemns people living with HIV to experience side effects of old medicines, which makes it less likely they will adhere to treatment – Souhaila Bensaid
“North Africa relies mostly on generic medicines for HIV treatment,” explains Souhaila Bensaid, Chair of Association ATP+ in Tunisia. “Our sub-region already registers the lowest coverage of HIV treatment on the continent. Such exclusions increase the treatment gap between countries in Africa. It condemns people living with HIV to experience side effects of old medicines, which makes it less likely they will adhere to treatment. This is particularly concerning in the context of early treatment initiation”.
“Yesterday, our colleagues in Morocco and Tunisia called on their governments to issue compulsory licenses on dolutegravir and to make sure that communities have access to generic medicines. This is a fundamental human right. Today, we as treatment and human rights advocates from all parts of the continent stand together; to request that ViiV and the Medicines Patent Pool review this current license and revise the provisions to ensure access for all Africans who require access to treatment”, concluded Michaela Clayton, Executive Director of ARASA.




