Today, 3 December 2015, the All-Ukrainian Network of People Living with HIV (the Network) hosted a roundtable meeting to explore how to improve access to antiretroviral drugs and achieve the 90-90-90 goal in Ukraine. 90–90–90 is an ambitious treatment target to help end the AIDS epidemic. Specifically the goals are that, by 2020:
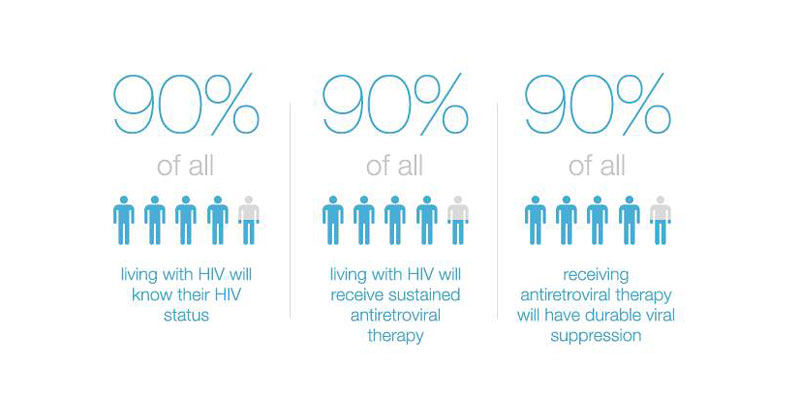
- 90% of all people living with HIV will know their HIV status,
- 90% of all people with diagnosed HIV infection will receive sustained antiretroviral therapy, and
- 90% of all people receiving antiretroviral therapy will have viral suppression.
 The meeting was attended by the Network Coordination Council members, Network’s central office staff, representatives of international organizations including WHO, USAID, US and CDC, national organizations and national experts.
The meeting was attended by the Network Coordination Council members, Network’s central office staff, representatives of international organizations including WHO, USAID, US and CDC, national organizations and national experts.
A range of issues were explored including: the need to expand HIV treatment programs; effective mechanisms for retaining people in HIV treatment; the need to reform the procurement and supply of medicines to treat HIV; and the need for qualitative structural changes to the existing infrastructure of health facilities and organizations involved in the HIV response in Ukraine.
As a result of the discussion the participants agreed that a common strategy is needed that should reflect the following fundamental questions:
Treatment
- The main task of the state and all partners must be rapid expansion of antiretroviral treatment over the next two years (2016-2017);
- For rapid expansion of treatment for people living with HIV, Ukraine should adapt the Test and Treat approach;
- Treatment should be available to all people who have been diagnosed (those who are registered and patients who have a diagnosis), with priority for patients with a CD4 count below 500;
- Separate strategies for treating different groups of patients are to be developed based on the WHO guidance (first diagnosed asymptomatic patients, first diagnosed patients with clinical symptoms, stable patients who receive treatment, unstable patients who receive treatment). For each of these subgroups specific interventions, meeting these patients’ needs, must be developed;
- Program indicators and intervention assessments should be improved. Monitoring and evaluation should demonstrate more closely the connection with the service cascade, and should focus on the indicators of impact on the epidemic;
- Optimization of treatment is needed, but it must be assessed not only in terms of the price of drugs, but also with regard to required quality, effectiveness and its ability to minimize side effects;
- It is necessary to improve the procurement and supply of medicines (framework agreements, synchronizing supply, creation of a buffer), as overload of the AIDS centers storage facilities could lead to loss and waste of drugs;
- Service delivery systems should include the gradual transfer of drug prescription functions (task shifting) from the specialized agencies to the primary care level. New approaches to retaining and re-entering patients under medical supervision should be offered in the first half of 2016.
Drugs
- There is an urgent need to develop a clear government policy to increase public funding and find alternative sources (donor funding) to cover the growing demand for antiretroviral drugs;
- Use of generic versions of old and new antiretroviral drugs (such as Integrase Inhibitors), taking into account medical indications and contraindications, should be part of the public policy in order to achieve greater economic efficiency;
- Inclusion of new drugs to the national treatment protocol should be based on the Health Technology Assessment, taking into account evidence-based medicine data, economic accessibility and cost-effectiveness of the drug in the long run (including the impact on the patients’ quality of life, the cost of treating adverse reactions and side effects, etc.);
- The results of new drugs (classes of drugs) should be considered when updating the National Essential Medicines List;
- It is necessary to accelerate the development of clinical guidelines and the national protocol, which should include approaches to HIV testing, use of drugs, and the application of CD4 and Viral Load tests to support treatment.
Working with stakeholders
- A large advocacy campaign should raise awareness of the country leadership and the Ministry of Health about the clinical and cost effectiveness of treatment programs, and the need to expand them;
- Effective information campaigns should be launched to inform health professionals and patients about the effectiveness, quality and safety of generic medicines (Tentative FDA Approval, WHO pre-qualification);
- Patients and health care staff should be informed about interactions of antiretroviral drugs with other medicines via electronic and printed materials;
- Activities to develop and support patients’ adherence to treatment is an essential component of treatment programs, and should be updated on a regular basis taking into account new challenges and tasks.




